Hypoxic-ischemic
encephalopathy
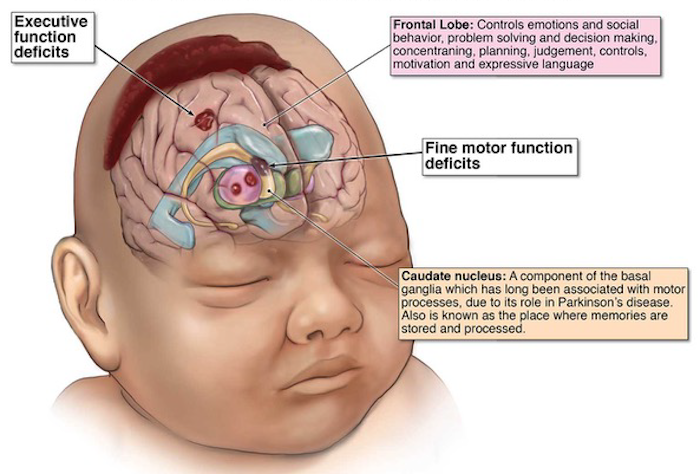
is a serious birth complication that causes obstruction in blood flow in brain
during the prenatal, intrapartum or postnatal period. This leads to death of the
child or mental disabilities in the first two years of age.
Access
Detailed Report Summary:
Although,
the cause of the disease is not yet identified but some conditions like cord
prolapse, abruptio placenta, placenta previa, uterine rupture, breech
presentation, maternal hypotension, or shoulder dystonia are known to be
observed in the patients with this disease. Some of the symptoms observed in
children with this disease are low heart rate, bluish skin colour, poor muscle
tone, and excessive acid in the blood.
Request
to Get the Sample Pages at:
Celgene
Corporation is in the process of developing HPDSC as a cell therapy for the
treatment of hypoxic-ischemic encephalopathy. Assiut University is in the
process of developing erythropoietin for the treatment of hypoxic-ischemic
encephalopathy. Further, GW Pharmaceuticals is also involved in the pipeline
for hypoxic-ischemic encephalopathy.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.








No comments:
Post a Comment