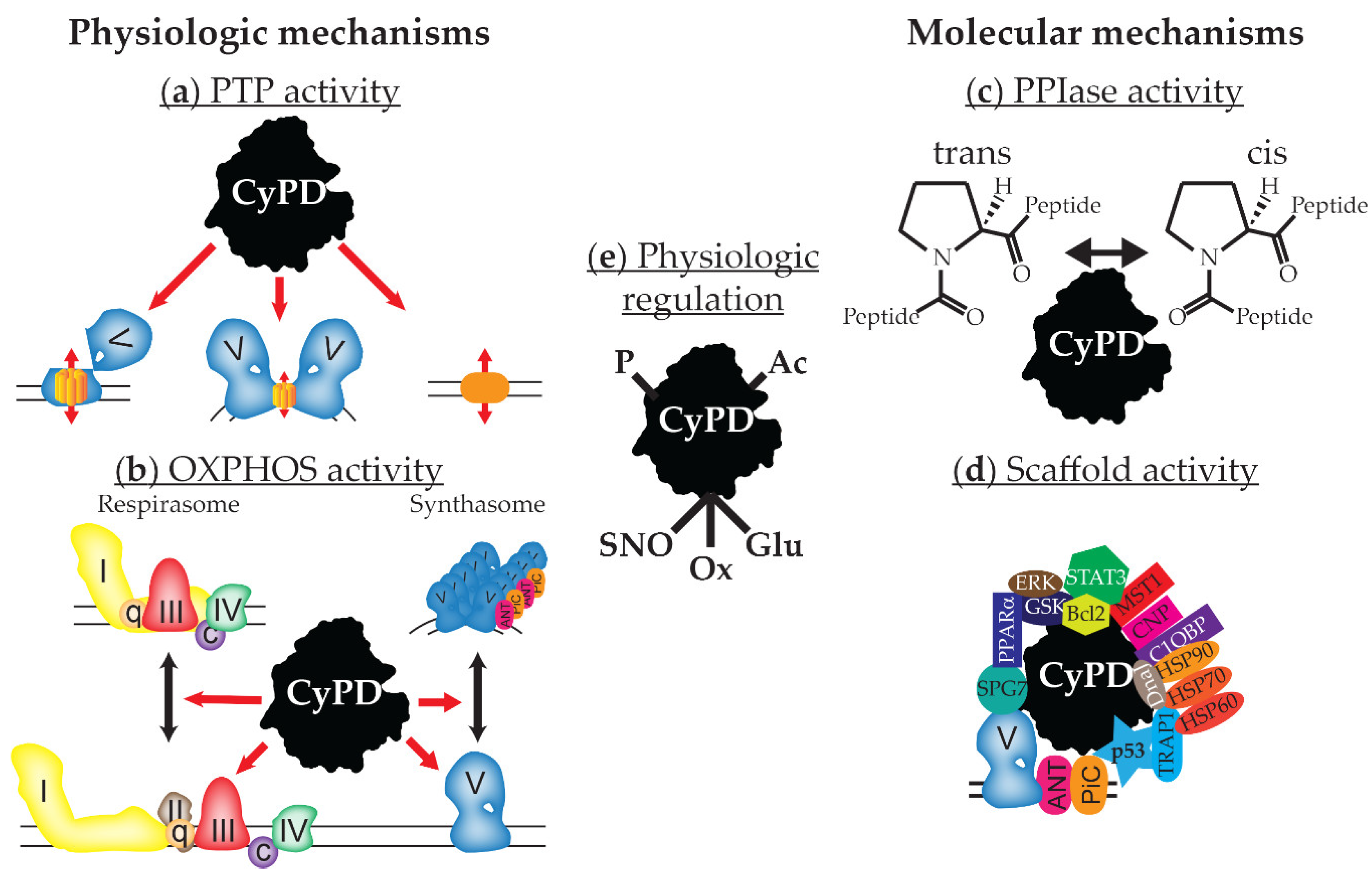
Hyperoxaluria Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr02:35allena hyperoxaluria, dcr-phxc, dicerna ldha, enteric hyperoxaluria prevalence, hyperoxaluria type 3, primary hyperoxaluria, primary hyperoxaluria type 1 mayo clinic, reloxaliase
No comments

Hyperoxaluria is a medical condition which
involves increased oxalate in urine. The causes of hyperoxaluria include
consumption of oxalate-rich foods, intestinal disorders and genetic disorders.
The disease is categorized into different types which includes primary
hyperoxaluria, enteric hyperoxaluria and hyperoxaluria related to eating
high-oxalate foods.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/report/hyperoxaluria-therapeutics-pipeline-analysis

The symptoms
observed during hyperoxaluria are severe back pain, blood in urine, frequent
urge to urinate, pain when urinating, and fever. The complications associated
with the hyperoxaluria includes kidney damage, decreased urine output, loss of
appetite, nausea, vomiting, pale skin colour related to anemia and swelling of
hands and feet. Hyperoxaluria is treated by using medications, high fluid
intake, dietary changes, dialysis and transplantation.
Report Description: https://www.pharmaproff.com/request-sample/1124
Allena
Pharmaceuticals Inc. is in the process of developing ALLN-177 as a non-absorbed
oral enzyme that specifically degrades oxalate for the treatment of
hyperoxaluria. Alnylam Pharmaceuticals Inc., and Dicerna Pharmaceuticals Inc.
are some other companies having pipeline drugs for hyperoxaluria.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Human Immunodeficiency Virus (HIV) Fusion Inhibitors Therapeutics - Pipeline Analysis, Clinical Trials & Results
Pramod Kmr02:29fusion inhibitors drug examples, fusion inhibitors hiv, fusion inhibitors mechanism of action, fusion inhibitors names, fusion inhibitors side effects, how do fusion inhibitors work
No comments

Human
immunodeficiency virus (HIV) fusion inhibitors is the new class of antiretroviral
drugs for the treatment of HIV infections. Failure of combination
antiretroviral therapy in patients with HIV, has increased the risk of disease
progression. This has led to the development of next generation of fusion inhibitor
peptides for better treatment of HIV. Thus, fusion inhibitors have emerged as
attractive therapeutics for the treatment of HIV infections.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1202
Studies
suggested that HIV fusion inhibitors offers potent antiretroviral activity but
its uptake has been limited because of the need for delivery by subcutaneous
injection administered twice-daily. Also, chances of cytotoxicity are low in
treatment with HIV fusion inhibitors therapeutics as they do not actually enter
the cells. Thus, providing many opportunities to the companies for the
development of new HIV fusion inhibitors with more potent activity and longer
half-lives for better therapeutics with reduced adverse events.
Report Description: https://www.pharmaproff.com/report/hiv-fusion-inhibitors
Frontier
Biotechnologies Inc. is in the process of developing Albuvirtide as a treatment-paradigm
shifting long-acting HIV fusion inhibitor for treatment of HIV infection. Mapp
Biopharmaceutical Inc., and United Biomedical Inc. are some other companies
having pipeline of HIV fusion inhibitors.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with detailed analysis of
pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licencing, grants, technology
and others.
Tau Protein Inhibitors Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr02:25and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Tau Protein Inhibitors Therapeutics - Pipeline Analysis 2018
No comments

Tau
protein is found
in abundance in neurons of the central nervous system. This protein is a
product of microtubule-associated protein tau (MAPT) gene, that is located on
chromosome 17. It is associated with pathologies of several nervous system
disorders, such as dementia, Parkinson’s disease, and Alzheimer’s disease.
The main
function of the protein is to stabilize microtubules. There are six isoforms of
the protein present in the brain tissue which can be distinguished by their
number of binding domains. The advancements in the protein by hyperphosphorylation
and aggregation are observed in a molecular study of the disease.
Report Description:
AC Immune SA
is developing a number of tau proteins inhibitor molecules. Neurimmune Holding
AG is also in the process of developing BIIB076 as a tau protein inhibitor, for
the treatment of Alzheimer’s disease. ProMIS Neurosciences Inc. is another
company developing tau proteins.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
Prader-Willi Syndrome – Epidemiology Insights
Pramod Kmr02:19prader willi syndrome chromosome, prader willi syndrome diagnosis, prader willi syndrome genetics, prader willi syndrome inheritance, prader willi syndrome life expectancy, prader willi syndrome treatment
No comments

Prader-Willi
syndrome (PWS)
is a complicated genetic condition which can affect various parts of the body.
During infancy, this condition is identified by weak muscle tone (hypotonia),
problems in feeding, poor growth, and delay in development.
Request
to Get the Sample Pages at:
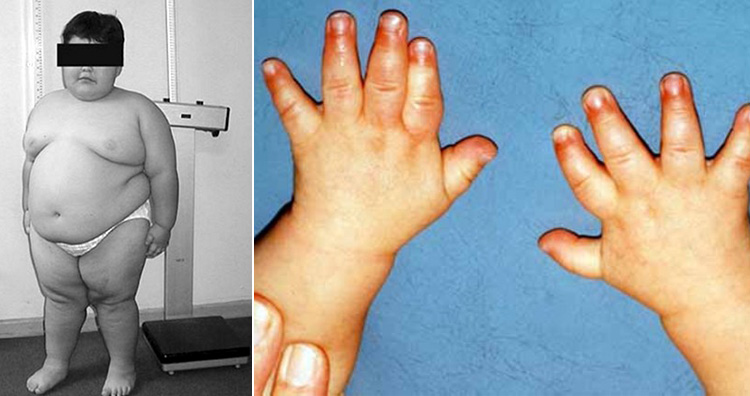
It begins in
childhood due to which the patients develop an insatiable appetite, which can result
in chronic overeating (hyperphagia) and obesity. Few patients with PWS,
especially those who are obese, also develop type 2 diabetes. Some common
symptoms of this disease are floppiness, learning difficulties, behavioral
problems, restricted growth, and excessive appetite or loss of appetite.
Report Description:
PWS is a
common form of obesity and affects about 350,000 and 400,000 individuals
worldwide. It affects males and females in equal ratio and occurs in all ethnic
groups and regions in the world.
The report
covers historical and forecast epidemiology of the disease in the seven major
markets including the U.S., EU5 (France, Germany, Italy, Spain, U.K.), and
Japan. The report has been compiled by building an understanding of the
disease, after reviewing numerous studies conducted by regulatory bodies in
various countries.
Glaucoma – Epidemiology Insights
Pramod Kmr02:14epidemiology of glaucoma, glaucoma articles, glaucoma pdf, glaucoma review, glaucoma statistics 2018, glaucoma statistics by country, open angle glaucoma prevalence, pathophysiology of glaucoma
No comments

Glaucoma can be described as a group of
diseases characterized by cupping of the optic nerve head and visual-field
damage. These diseases differ from each other in terms of their causes, risk
factors, demographics, symptoms, duration, treatment, and prognosis.
Report Description:

The common
identifiable features for all forms of glaucoma are loss of retinal ganglion
cells, thinning of the retinal nerve fiber layer, and cupping of the optic
nerve. There are two key categories of glaucoma; open-angle glaucoma (OAG), and
narrow angle glaucoma.
Request
to Get the Sample Pages at:
Glaucoma is
mainly associated with old age and its overall prevalence is found to be
significantly lower in regions with young population. The epidemiological
studies have reported that the global prevalence of glaucoma is approximately
3.5% for people aged 40-80 years.
The report
covers historical and forecast epidemiology of the disease in the seven major
markets including the U.S., EU5 (France, Germany, Italy, Spain, U.K.), and
Japan. The report has been compiled by building an understanding of the
disease, after reviewing numerous studies conducted by regulatory bodies in
various countries.
Hyperkalemia Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations
Pramod Kmr22:34and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hyperkalemia Therapeutics - Pipeline Analysis, Patents
No comments

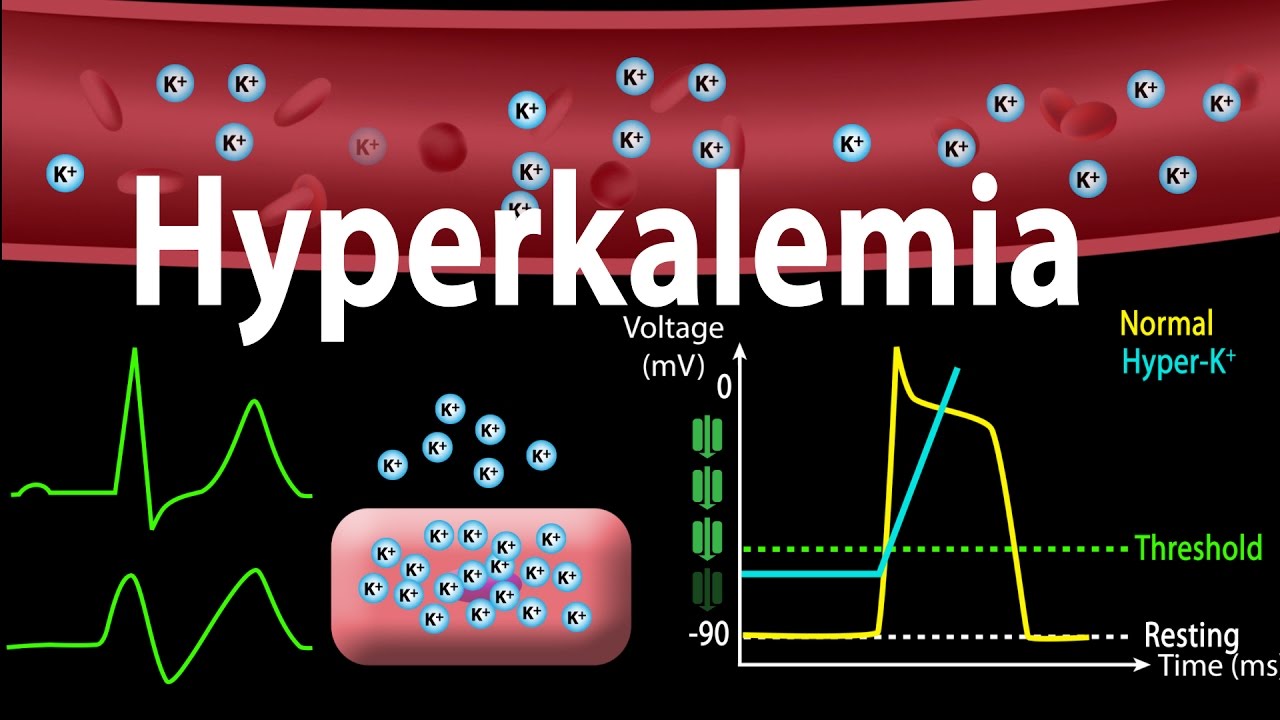
Hyperkalemia is referred to as increased levels
of potassium in blood which results in cardiac arrest and death. Potassium
levels above 5.1 mEq/l are considered as the hyperkalemia condition. The main
causes of hyperkalemia are potassium sifting out of cells into the blood circulation,
adrenal gland diseases, kidney dysfunction, uncontrolled diabetes, breakdown of
muscle tissue and red blood cells.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/hyperkalemia-therapeutics-pipeline-analysis
Hyperkalemia
causes abnormal heart rhythms and also interferes in the functioning of the
skeletal muscles. The common symptoms associated with the hyperkalemia are
fatigue, nausea, muscle weakness and tingling sensations.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1155
The standard
treatment strategies for hyperkalemia are intravenous administration of glucose
and insulin, a diet low in potassium, medications that stimulate beta-2 adrenergic
receptors, sodium bicarbonate administration and discontinue medications that
increase blood potassium levels. Ardelyx Inc. is developing RDX013 which acts
as a potassium secretagogue for the treatment of hyperkalemia. Relypsa Inc. is
another key player involved in the development of drugs for the treatment of
hyperkalemia.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Wiskott-Aldrich Syndrome Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents, Designations
Pramod Kmr22:31and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Wiskott-Aldrich Syndrome Therapeutics - Pipeline Analysis
No comments

Wiskott-Aldrich
syndrome is a
hereditary immunodeficiency disorder characterized by abnormal functioning of
immune system and decreased ability to form blood clots. The abnormality in the
platelets leads to easy bruising effects in case of prolonged bleeding.
The white
blood cells become non-functional and cause other immunological disorders in
Wiskott-Aldrich syndrome. It has been discovered that this disease is caused by
the mutation in WAS gene (Xp11.4-p11.21) that instruct to make Wiskott-Aldrich
syndrome protein. Some of the symptoms observed in the patients are
thrombocytopenia, hematologic abnormalities, eczema, and malignancies.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1152
Genethon
Inc. is in the process of developing autologous CD34 positive cells transduced
with a lentiviral vector containing human WAS gene as a gene therapy for the
treatment of Wiskott-Aldrich syndrome. Further, the University of Pittsburgh
and Orchard Therapeutics Limited are also involved in the pipeline for
Wiskott-Aldrich syndrome.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Vaginitis Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations
Pramod Kmr22:21and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Vaginitis Therapeutics - Pipeline Analysis
No comments

Vaginitis refers to the inflammation of
vagina. It affects women of all ages but is most common during the reproductive
years. Vaginitis occurs due to change in the balance of the yeast and bacteria
that normally live in the vagina.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/vaginitis-therapeutics-pipeline-analysis

This causes
the lining of the vagina to become inflamed. Use of antibiotics, changes in
hormone levels due to pregnancy, breastfeeding, menopause, douching,
spermicides, sexual intercourse and infection can change the normal balance of
the vagina. The main symptom of vaginitis is increased discharge with a strong
fishy odour. Itching is not common, but may be present if there is a lot of
discharge.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1151
Vaginitis
can be diagnosed by taking a sample of the discharge from vagina, which is then
observed under a microscope. The treatment depends on the cause of the
vaginitis. It may include a pill or a cream or gel that is applied to the
vagina.
The drug
candidates in vaginitis pipeline include, but are not limited to, Polygynax and
Tamoxifen vaginal. Some of the companies in vaginitis therapeutics pipeline are
Lumavita AG and Fulhold Pharma Limited.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Uterine Fibroids Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr01:31and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Uterine Fibroids Therapeutics - Pipeline Analysis
No comments

Uterine
fibroids, also
known as leiomyomas or myomas, are non-cancerous growths that develop from the
muscle tissue of the uterus. The size, shape, and location of fibroids can vary
greatly. They may be present inside the uterus on its outer surface, or within
its wall, or attached to it by a stem-like structure.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/uterine-fibroids-therapeutics-pipeline-analysis

A fibroid
may remain very small for a long time and suddenly grow rapidly, or grow slowly
over a number of years. It usually affects women between the age of 30 to 40
years, but can occur at any age. Uterine fibroids may have various symptoms,
such as change in menstruation, anaemia, abdominal cramps, constipation, pain
during sex, rectal pain, and difficulty urinating or frequent urination. It is
diagnosed through ultrasonography, hysteroscopy, hysterosalpingography, and
sonohysterography. Some medications are known to reduce heavy bleeding and
painful periods, caused due to this condition.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1150
The drug
candidates in uterine fibroids disease pipeline include, but are not limited
to, Elagolix, OBE2109, and Proellex. Some of the companies having drugs in the
uterine fibroids disease pipeline are Neurocrine Biosciences Inc., Abbvie Inc.,
and ObsEva SA.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Turner Syndrome Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr01:27and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Turner Syndrome Therapeutics - Pipeline Analysis
No comments

Turner
syndrome is a
chromosomal condition, which occurs when one of the two X chromosomes normally
found in women is missing or incomplete. The disease alters the growth and
development in females. The symptoms can vary among women who have Turner
syndrome. Women with this condition tend to be shorter than average and are
usually unable to conceive a child because of an absence of ovarian function.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/turner-syndrome-therapeutics-pipeline-analysis

Some other
features of this condition are extra skin on the neck (webbed neck), heart
defects and kidney problems, puffiness or swelling (lymphedema) of the hands
and feet, and skeletal abnormalities. This condition occurs in about one in
2,500 female births worldwide, but is much more common among pregnancies that
do not survive to term (miscarriages and stillbirths).
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1149
The drug
candidates of Turner syndrome disease pipeline include, but are not limited to
are Estradiol and Somatropin pegylated. Some of the companies having drugs in
the Turner syndrome disease pipeline are Novo Nordisk A/S, GeneScience
Pharmaceuticals Co. Ltd., and Polus Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Reperfusion Injury Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr01:21and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Reperfusion Injury Therapeutics - Pipeline Analysis 2018
No comments

Reperfusion injury is the damage to tissues
caused when blood supply returns to the tissue after a period of ischemia or
lack of oxygen (anoxia, hypoxia). It influences the patient’s outcome after
myocardial infarction, stroke, cardiovascular surgery, and organ
transplantation.
Access
Detailed Report Summary:
The
treatments available for reperfusion injury are therapeutic hypothermia,
hydrogen sulfide treatment, cyclosporin, stem cell therapy and metformin.
Radikal Therapeutics Inc. is in the process of developing R-190 as an
intravenous formulation for the treatment of limb ischemia reperfusion injury.
The drug candidate is being developed as a small molecule which acts as a
nitric oxide donors and reactive oxygen species inhibitor. Some of the
companies having a pipeline of reperfusion injury include Catalyst Biosciences
Inc., MIFCOR Inc. and others.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1148
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Post-Operative Nausea and Vomiting Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents, Designations
Pramod Kmr02:35and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Post-Operative Nausea and Vomiting Therapeutics - Pipeline Analysis 2018
No comments

Post-operative
nausea and
vomiting is one of the complications of anaesthesia given to the patients at
the time of a surgery. The state of having unpleasant sensation subjected to
desire of vomiting is known as nausea whereas, vomiting is the forceful
expulsion contents present in gastrointestinal tracts.
Access
Detailed Report Summary:
https://www.pharmaproff.com/report/post-operative-nausea-and-vomiting-therapeutics-pipeline-analysis
The
chemoreceptor trigger zone, vagal mucosal pathway, neuronal pathway, reflex
afferent pathway, and midbrain afferent pathway are responsible for the
stimulation of vomiting. These pathways stimulate histaminergic, cholinergic,
and dopaminergic receptors to activate the sensation of vomiting. In the
postrema region, neurokinin-1 receptors are present which also play an
important role in emesis (action of vomiting).
Request
to Get the Sample Pages at:
Camurus AB
is in the process of developing CAM2058 as a serotonin 3 receptor antagonist
for the treatment of post-operative nausea and vomiting. Some of the companies
and universities having the pipeline of post-operative nausea and. Further,
universities like the University of British Columbia and Assiut University are
also involved in the pipeline for post-operative nausea and vomiting.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline analysis
of drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licensing, grants, technology, and others.
Mucopolysaccharidosis III (MPS III) (Sanfilippo Syndrome) Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results
Pramod Kmr02:30and Other Developments, Clinical Trials & Results, Collaborations, Designations, Mucopolysaccharidosis III (MPS III) (Sanfilippo Syndrome) Therapeutics - Pipeline Analysis 2018, Patents
No comments

Mucopolysaccharidosis
type III (MPS III), also known as Sanfilippo syndrome, is a progressive disorder that
primarily affects brain and spinal cord (central nervous system). People with
MPS III generally do not display features at birth, but they begin to show
signs and symptoms of this disorder during early childhood. Affected children often
initially have delayed speech and behaviour problems. In later stages of this
disorder, people with MPS III may develop seizures and movement disorders.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/mps-iiitherapeutics-pipeline-analysis
Patients
with MPS III are also known to have short stature, joint stiffness, or mild
dysostosis multiplex. Affected individuals often experience chronic diarrhoea
and recurrent upper respiratory and ear infections. People with MPS III may
also have hearing loss and vision problems. MPS III is divided into four types;
IIIA, IIIB, IIIC, and IIID, which are distinguished by their genetic cause.
Request
to Get the Sample Pages at:
The drug
candidates in MPS III pipeline include, but are not limited to, EGT-101, MPS
IIIA (Sanfilippo A) Program and LYS-SAF302. Some companies having drugs in the
MPS III pipeline are Esteve Pharmaceuticals S.A., Orchard Therapeutics Limited,
Lysogene S.A. among others.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Viral Bronchiolitis Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr02:25and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Viral Bronchiolitis Therapeutics - Pipeline Analysis 2018
No comments

Viral
bronchiolitis is
an inflammatory disorder which is characterized by obstruction of small
airways, necrosis and edema of the epithelial cells, and increased mucus
production. The disease is most commonly caused by an infection caused by
respiratory syncytial virus.
Access
Detailed Report Summary:
The signs
and symptoms observed in the patients are low grade fever, congestion, and
apnea. However, in most severe cases, respiratory distress, cyanosis, and
irritability also occurs in the patients. Viral bronchiolitis is the most
common illness observed in children in their first two years of life.
Request
to Get the Sample Pages at:
Ait
Therapeutics Inc. is in the process of developing AIT-RSV as a guanylate
cyclase stimulant for the treatment of viral bronchiolitis. Some of the other
companies and universities having pipeline drugs for viral bronchiolitis
include Washington University School of Medicine, and Vaxart Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Pleural Effusion Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results
Pramod Kmr01:29and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Pleural Effusion Therapeutics - Pipeline Analysis 2018
No comments

Pleural
effusion is the
fluid build-up in the pleural space and is categorized into two types which
includes exudate and transudate. Transudate is usually composed of
ultrafiltrates of plasma due to heart failure or cirrhosis; while, exudate is
caused by inflammatory conditions. The major causes of pleural effusion are
kidney failure, infection, congestive heart failure, malignancy, pulmonary
embolism, cirrhosis, hypoalbuminemia and trauma.
The common
symptoms associated with the pleural effusion are chest pain, difficulty in
breathing, painful breathing, cough, fever and loss of appetite. The standard
treatments available for pleural effusion are thoracentesis, surgery,
antibiotics and diuretics.
Request
to Get the Sample Pages at:
Lung
Therapeutics Inc. is in the process of developing LTI-01 as a single chain
urokinase plasminogen activator for the treatment of safe clearance of
fibrinous scar tissue in patients with loculated pleural effusion. Genelux
Corporation is another key player involved in the development of drugs for the
management of pleural effusion.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Interstitial Lung Disease Therapeutics - Pipeline Analysis
Pramod Kmr01:24and Other Developments, Clinical Trials & Results, Collaborations, Designations, Interstitial Lung Disease Therapeutics - Pipeline Analysis 2018, Patents
No comments

interstitium
of the lungs. The interstitium is a lace like network of the tissue that
provide support to the air sacs and the capillaries in the interstitium which
allows the gas exchange between the blood and alveolar tissues.

Some of the
types of the condition include interstitial pneumonia, idiopathic pulmonary
fibrosis, and non-specific interstitial pneumonitis. Major symptoms associated
with the disease include dry cough and shortness of breath.
Request
to Get the Sample Pages at:
Regend
Therapeutics is in the process of developing lung stem cells as a cell therapy
for the treatment of interstitial lung disease. Boehringer Ingelheim GmbH is in
the process of developing nintedanib as a fibroblast growth factor receptor
antagonist for the treatment of this medical condition.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Nav1.7 Voltage-Gated Sodium Channel Inhibitors Therapeutics - Pipeline Analysis
Pramod Kmr01:21and Other Developments, Clinical Trials & Results, Collaborations, Designations, Nav1.7 Voltage-Gated Sodium Channel Inhibitors Therapeutics - Pipeline Analysis 2018, Patents
No comments

Nav1.7
voltage-gated sodium channel inhibitors are the drug candidates that target the sodium
channels Nav1.7 and have been significant in pain management. The sodium
channels Nav1.7 receptors are generally found in two types of neurons:
nociceptive pain neurons, such as trigeminal neurons and dorsal root ganglion;
and sympathetic ganglion neurons which form a part of the autonomic
(involuntary) nervous system. These receptors play an important role in
generation and conduction of action potential.

The sodium
channels Nav1.7 is generally encoded by SCN9A gene. Voyager Therapeutics Inc.
is developing VY-NAV01 as a Nav1.7-voltage-gated-sodium-channel-inhibitor for
the treatment of severe and chronic pain. Sumitomo Dainippon Pharma Co. Ltd. is
in the process of developing DSP-2230 as a Nav1.7 and Nav1.8
voltage-gated-sodium-channel-inhibitor for the treatment of neuropathic pain.
Some of the companies having the pipeline of Nav1.7 voltage-gated sodium
channel inhibitors include Xenon Pharmaceuticals Incorporated, SiteOne
Therapeutics Inc., Icagen Inc. and others.
Request
to Get the Sample Pages at:
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
RNA Interference (RNAi) Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr02:13and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, RNA Interference (RNAi) Therapeutics - Pipeline Analysis 2018
No comments

RNA
interference (RNAi) is a molecule type that silences the gene and limits the transcription
of the mutated gene. Gene silencing is a novel mechanism that inactivates the
transcripts of mutated gene, by activating sequence specific RNA degradation
process. The process of RNAi is also known as post-transcriptional gene
silencing. This novel mechanism holds potential to revolutionize the biological
science for escaping out the disease.
The drug candidate
acts upon double stranded DNA or double stranded RNA. There are several
applications of RNAi in various areas, such as gene knockdown, functional
genomics, medicine, biotechnology, and genome scale screening. In medicine, the
therapeutic candidate can be used for the treatment of virus-related disease
and cancer, whereas, in biotechnology, this novel mechanism can help in the
production of transgenic plants and nutrient enriched food.
Request
to Get the Sample Pages at:
Dicerna
Pharmaceuticals Inc. is developing a wide range of next generation RNAi
therapies in clinical and non-clinical stages of development. Some of the
companies having pipeline of RNAi therapies include Silence Therapeutics plc,
Arrowhead Research Corporation Inc., and Phio Therapeutics Corp.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
Rho Kinase Inhibitor Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations
Pramod Kmr02:01and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Rho Kinase Inhibitor Therapeutics - Pipeline Analysis
No comments

Rho kinase
inhibitor, also
known as ROCK inhibitor, inhibits the rho kinases, a family of small GTP-binding
protein. Rho kinases are found to regulate cell motility, proliferation, shape,
gene expression, apoptosis, and are also involved in the signalling pathway.
The inhibition of rho kinases has shown some beneficial effects in
cardiovascular diseases, including systemic hypertension, vasospastic angina,
stable effort stigma, pulmonary hypertension, stroke, and heart failure.
Rho kinases
are a member of serine/threonine kinases and are encoded by ROCK1 and ROCK2
gene. There are many evidences that show rho kinase inhibitors play a
significant role in improving symptoms related to cardiovascular diseases, as
it has been experimentally proven that rho kinase gets upregulated in the
cardiovascular disorders. The development of inhibitors of rho kinase isoforms can
be an advantage, inclined to the specificity of the cardiovascular disorders.
In addition, the pathophysiology of other disorders, such as intracerebral
hemorrhage, diabetes, Parkinson’s disease, and Alzheimer’s disease are related
to the modulation of rho kinases.
Request
to Get the Sample Pages at:
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
p53 Antigen Modulators Therapeutics - Pipeline Analysis, Clinical Trials & Results, Patents, Designations
Pramod Kmr01:49and Other Developments, Clinical Trials & Results, Collaborations, Designations, p53 Antigen Modulators Therapeutics - Pipeline Analysis 2018, Patents
No comments

p53 is the tumor suppressor
transcription factor that activates to various stimulus, including uncontrolled
cell proliferation, oncogene over-expression, and DNA damage. p53 antigen
modulators helps in preventing cancer development through regulation of cell
cycle and apoptosis. Thus, new opportunities in cancer immunotherapy are
expected to develop better drug candidates targeting p53, with complete understanding
of interactions between p53 and the immune system, to avoid various adverse
events. The major challenge is prevention of tissue damage by selectively
modulating p53 activity, under pathophysiological conditions that generate
redox stress.
p53 antigen
modulator therapies are being widely studied for the development of various
target specific cancer therapies. Dendritic cell-derived vaccines, adenoviral
p53 vectors, MDM2 inhibitors and small-molecules to reinstate the DNA binding
activity of p53 are some commercial approaches as p53 antigen modulator
therapies, for the treatment of various indications. Also, research studies
have demonstrated that novel therapeutic strategies are being developed to
overcome the challenges related to in-depth knowledge of p53 and associated
pathways.
Request
to Get the Sample Pages at:
Quark
Pharmaceuticals Inc. is developing QPI-1002 as a nuclease-resistant, synthetic
double-stranded RNA oligonucleotide designed to temporarily inhibit the
expression of the pro-apoptotic gene p53, for the treatment of delayed graft
function and acute kidney injury. Some of the companies having a therapeutic
pipeline of p53 antigen modulators include Innovation Pharmaceuticals Inc.,
Aprea Therapeutics, and Actavalon Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with detailed analysis of
pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licencing, grants, technology
and others.
Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Inhibitor Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr01:37and Other Developments, Clinical Trials & Results, Collaborations, Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Inhibitor Therapeutics - Pipeline Analysis 2018, Designations, Patents
No comments

Cytotoxic
T-lymphocyte antigen-4 (CTLA-4) plays important role in the T-cell regulation at an early
stage of naive T-cell activation, primarily in the lymph nodes. CTLA-4 has
emerged as an attractive cancer immunotherapy as a part of “immune checkpoint
blockade”. CTLA-4 pathway inhibitors enhance T-cell activation and amplify
T-cell proliferation. Successful results of immune checkpoint inhibitors in
cancer immunotherapy has led to the development of many new agents and
strategies, including combination for the treatment of various cancers.
Despite of
many advancements in the development of anti-cancer therapies, cancer is still
one of the major causes of deaths, globally. Combination therapies are proving
to be more efficient than monotherapy for the treatment of cancer. Studies have
demonstrated that concurrent PD-1 and CTLA-4 blockade have shown positive
results in patients with advanced melanoma. This provides huge opportunities
for development of better combinatorial immunotherapeutic therapies for
checkpoint blockade with molecular targeted therapies, angiogenesis inhibition
and novel vaccines.
Request
to Get the Sample Pages at:
Agenus Inc.
is in the process of developing AGEN1884 as a monoclonal antibody which acts as
a CTLA-4 inhibitor for the treatment of non-small cell lung cancer. It is also
being studied in combination with pembrolizumab, for the treatment of cancer.
Some of the other companies having pipeline of CTLA-4 inhibitor include
AstraZeneca PLC, ImmunOs Therapeutics AG, Tikcro Technologies Ltd.
Cyclophilin Inhibitors Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents, Designations
Pramod Kmr01:10Clinical Trials & Results, Collaborations, Cyclophilin Inhibitors Therapeutics - Pipeline Analysis, Designations, Other Developments, Patents
No comments

Cyclophilin befits in the group of protein that
shows peptidyl-prolyl cis-trans isomerase activity, found in all types of
cells. In humans, 16 cyclophilins have been identified till date. Cyclophilin
A, a member of cyclophilin group, mediate the action of immunosuppressive
drugs. A ternary complex is formed when cyclophilin A interact with another
cyclosporin A and inhibits calcineurin protein that regulates cytokine gene
transcription.
Cyclophilin A is involved in trafficking of proteins that
distributes action of asialoglycoprotein receptor between plasma membrane and
endosomal pool; promotes nuclear export; and translates neuronal nuclei to
induce cell death in various pathological conditions which includes amyotrophic
lateral sclerosis and cerebral hypoxia-ischemia. In addition, cyclophilin plays
important role in cell signalling. Cyclophilin D is located in mitochondria and
regulates the opening of mitochondrial permeability transition pore.
Cyclophilins are involved in pathophysiology of neurodegenerative diseases.
Request to Get the Sample Pages at:
Several
companies, such as Ensemble Therapeutics, Debiopharm Group, and ContraVir
Pharmaceuticals Inc., are actively involved in the development of cyclophilin
inhibitors therapeutics. For instance, Ensemble Therapeutics is in the process
of developing a drug candidate that acts as a cyclophilin antagonist for the
treatment of Parkinson’s disease, amyotrophic lateral sclerosis, and hepatitis
B. Debiopharm Group is developing Debio 025 cyclophilin inhibitor for the
treatment of muscular dystrophy. Moreover, ContraVir Pharmaceuticals Inc. is
also developing CRV431 as a cyclophilin antagonist for the treatment of
hepatitis B.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
Cell Cycle Inhibitors Therapeutics - Pipeline Analysis , Clinical Trials and Results Report
Pramod Kmr01:05and Other Developments, Cell Cycle Inhibitors Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Collaborations, Designations, Patents
No comments

Cell
cycle inhibitors
include cyclin inhibitors and cyclin-dependent kinases (CDKs), which plays
major role in developing new class of anti-cancer therapies. Also, cell cycle
inhibitors in combination with chemotherapy, overcome drug resistance and
improve cytotoxic efficacy. CDKs are rational targets for cancer treatment,
that could restore cell-cycle checkpoints and may induce apoptosis.
Tolero
Pharmaceuticals, Inc., a clinical-stage company focused on developing novel
therapeutics for hematologic and oncologic disorders, developing alvocidib - a
potent CDK9 inhibitor in combination with cytarabine and daunorubicin. In April
2018, the company presented preclinical data supporting the apoptosis-inducing
activity of alvocidib at the American Association for Cancer Research (AACR)
Annual Meeting in the U.S., Chicago.
Request
to Get the Sample Pages at:
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with detailed analysis of
pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licencing, grants, technology
and others.
Androgen Receptor Antagonist Therapeutics - Pipeline Analysis, Clinical Trials & Results
Pramod Kmr00:59and Other Developments, Androgen Receptor Antagonist Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Collaborations, Designations, Patents
No comments

Androgens are responsible for the development
of male characteristics by binding to androgen receptors. Androgen receptor
plays an important role in the development and progression of prostate cancer.
Thus, it has been an attractive target for the clinical interventions and treatment
of prostate cancer. This has led to the development of new therapies for the
better treatment of castration-resistant prostate cancer.
Several
studies have demonstrated the promising results of cancer immunotherapy with
androgen receptor antagonists for the treatment of prostate cancer. Androgen
receptor antagonists with immunotherapy enhanced the immune response and
prevent the relapse of advanced prostate cancer. Thus, providing many
opportunities to the companies for the development of combination therapies of
androgen receptor antagonists with immunotherapy for the treatment of prostate
cancer.
Request to Get the Sample Pages at:
Taiho
Pharmaceutical Co. Ltd. is in the process of developing TAS3681 as a novel oral
androgen receptor antagonist with dual mechanism of action, acting as both an
androgen receptor antagonist and a down-regulator of androgen receptor, for the
treatment of prostate cancer. Pfizer Inc., Eisai Co., Ltd., and Evgen Pharma
Limited are some other companies having pipeline of androgen receptor
antagonist.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with detailed analysis of
pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licencing, grants, technology
and others.
Androgen Receptor Antagonist Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents
Pramod Kmr03:34and Other Developments, Androgen Receptor Antagonist Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Collaborations, Designations, Patents
No comments

Androgens are responsible for the development
of male characteristics by binding to androgen receptors. Androgen receptor
plays an important role in the development and progression of prostate cancer.
Thus, it has been an attractive target for the clinical interventions and
treatment of prostate cancer. This has led to the development of new therapies
for the better treatment of castration-resistant prostate cancer.
Several
studies have demonstrated the promising results of cancer immunotherapy with
androgen receptor antagonists for the treatment of prostate cancer. Androgen
receptor antagonists with immunotherapy enhanced the immune response and
prevent the relapse of advanced prostate cancer. Thus, providing many
opportunities to the companies for the development of combination therapies of
androgen receptor antagonists with immunotherapy for the treatment of prostate
cancer.
Request
to Get the Sample Pages at:
Taiho
Pharmaceutical Co. Ltd. is in the process of developing TAS3681 as a novel oral
androgen receptor antagonist with dual mechanism of action, acting as both an
androgen receptor antagonist and a down-regulator of androgen receptor, for the
treatment of prostate cancer. Pfizer Inc., Eisai Co., Ltd., and Evgen Pharma
Limited are some other companies having pipeline of androgen receptor
antagonist.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with detailed analysis of
pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licencing, grants, technology
and others
Alpha-Synuclein Inhibitor Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents, Designations
Pramod Kmr03:30Alpha-Synuclein Inhibitor Therapeutics - Pipeline Analysis 2018, and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents
No comments

Alpha-synuclein is a protein that is abundantly
found in the brain. The minimal amount of alpha-synuclein is also found in the
heart and other tissues. The protein is prominently found at the tips of the
nerve cells. It helps in the maintaining supply of the synaptic vesicle in
presynaptic terminals.
In addition,
the alpha-synuclein protein also helps in release of dopamine. The protein
comprised of 140 amino acids and is encoded by Synuclein Alpha (SNCA) gene. It
produced mostly in hippocampus, neocortex, substantia nigra, and cerebellum.
However, the actual function of alpha-synuclein is still completely unknown.
However, the pathophysiology of Parkinson’s disease is associated with the
accumulation of alpha-synuclein protein which forms Lewy bodies.
Request to Get the Sample Pages at:
F.
Hoffmann-La Roche Ltd. is in the process of developing RG7935 as a monoclonal
antibody targeting alpha-synuclein, for the treatment of Parkinson’s disease.
AC Immune SA is in the process of developing morphomer α-syn as an
alpha-synuclein inhibitor for the treatment of Parkinson’s disease. Neuropore
Therapies Inc. is also developing a drug candidate that targets alpha-synuclein,
for the treatment of Parkinson’s disease and multiple system atrophy.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials. Pipeline analysis of drugs by phases includes
product description and development activities including information about
clinical results, designations, collaborations, licensing, grants, technology,
and others.
Dasatinib - Drug Insight Insights, Potential Business Strategies, Mergers and Acquisitions, Revenue Analysis
Pramod Kmr03:26Dasatinib - Drug Insight Demand, Dasatinib - Drug Insight Growth, Dasatinib - Drug Insight Outlook, Dasatinib - Drug Insight Share, Dasatinib - Drug Insight Size
2 comments

Dasatinib (Sprycel) medicines are generally used to
treat people with chronic myeloid leukemia and people with acute lymphoblastic
leukemia who have been tested positive for the Philadelphia chromosome (Ph+).
Developed by Bristol-Myers Squibb Company, Dasatinib has been approved by the
FDA for the treatment of pediatric patients aged one-year-old with newly
diagnosed Ph+ acute lymphoblastic leukemia in combination with chemotherapy.
The clinical trials have shown positive results with increased safety and
efficacy. The most common side-effects of Dasatinib in children with
chemotherapy include swelling, pain, fever, nausea, and diarrhea.
The report
provides a comprehensive understanding of the drug, covering all the API
manufactures and its details in the 7 major markets which includes EU5 (U.K.,
Spain, Germany, Italy and France), U.S., and Japan. It covers all patents and
strategic developments reported in this drug area. It highlights the historical
and forecasted sales along with the market scenario, market competition and the
historical and emerging therapies. The report is inclusive of SWOT and PESTLE
analysis, depending on the information availability.
Request to Get the Sample Pages at:
Moreover,
the report also provides a comprehensive understanding of the pipeline
activities of the drug candidate under various stages of development, with
detailed analysis of pipeline and clinical trials. Pipeline analysis of drug by
phases includes product description and development activities including
information about clinical results, designations, collaborations, licensing,
grants, technology and others.