Acute Kidney Injury (AKI) Therapeutics Segmental Analysis by Therapeutics, Diagnostics, Patient, Drugs Policy and Regulatory Landscape
Pramod Kmr00:512019 - Pipeline Analysis, Acute Kidney Injury (AKI) Therapeutics, and Other Developments, Clinical Trials and Results, Collaborations, Designations, Patents
No comments

According to
a new research report “Acute
Kidney Injury (AKI) Therapeutics - Pipeline Analysis 2019, Clinical
Trials and Results, Patents, Designations, Collaborations, and Other
Developments” published by Pharma Proff, AKI therapeutics currently exhibits a proliferating
pipeline of 39 therapeutic candidates.
AKI or acute
kidney failure is a common medical condition reported among hospitalized
patients, especially those with multiple comorbid conditions. AKI can occur due
to various reasons including decreased renal perfusion, and obstruction of the
urinary collecting system. Dehydration, abdominal pain, rise in blood pressure,
mild back pain, and vomiting are some of the symptoms associated with the
disease.
According to
the research findings, majority of pipeline drug candidates are being developed
for intravenous route administration. The advantage with this administration
includes entire administered dose reaches the systemic circulation immediately,
which increases the bioavailability of drug. Administration of therapeutics for
AKI through intravenous route has shown promising results in clinical studies.
In November
2018, Angion Biomedica Corp. and Sinovant Sciences HK Limited entered into a
collaboration and license agreement for the development of ANG-3777 for the
treatment of patients with delayed graft function (DGF) following kidney
transplantation and AKI following open-heart surgery requiring cardiopulmonary
bypass.
Similarly,
in May 2016, A1M Pharma AB collaborated with CSL Behring LLC to investigate the
potential of combination therapy of Alpha 1 Microglobulin (A1M) and plasma
protein in patients suffering from pre-eclampsia and AKI. The endogenous
protein A1M is expected to showcase several protective mechanisms in tissues.
Sentien
Biotechnologies Inc., AM-Pharma B.V., and Quark Pharmaceuticals Inc., are some
other major companies involved in the development of drug candidates for the
treatment of AKI.
AKI
Therapeutics Pipeline Analysis
By Phase
By Molecule
Type
By Route of
Administration
By Company
Achondroplasia Therapeutics Pipeline Analysis -Drug Profile, Top Industry Intelligence and Therapeutic Development
Pramod Kmr00:44Achondroplasia Therapeutics - Pipeline Analysis 2018, and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents
No comments

Achondroplasia
therapeutics currently exhibits a considerably strong pipeline with seven
active drug candidates.
Achondroplasia is a common disorder of dwarfism
with disproportionate stature. It is an autosomal dominant disorder that occurs
due to mutation in fibroblast growth factor receptor 3 (FGFR3) gene, which is
located on short arm of chromosome 4. The disorder is characterized by
macrocephaly, shortened-limbs (rhizomelic dwarfism), midface retrusion, and
frontal bossing. The achondroplasia therapeutics pipeline study covers seven
active drug candidates in different stages of development.
Access
Detailed Report Summary:
Achondroplasia
therapeutics pipeline has majority of candidates in the Pre-Clinical stage of
development. A peptide-based drug candidate namely, vosoritide, is in the Phase
III stage of development by BioMarin Pharmaceutical Inc., for the treatment of
achondroplasia.
The drug
candidates developed by several companies for the treatment of achondroplasia
have shown positive clinical results in the various phases of drug development.
For instance, BioMarin Pharmaceutical Inc.’s drug candidate vosoritide, which
is under the Phase III stage of development, has been well-tolerated at all
doses in the Phase II stage’s study. The majority of adverse events (AEs) were
mild and no serious AEs were reported during the study. Across all doses,
injection site reactions and hypotension were the most common drug-related AEs.
Request
to Get the Sample Pages at:
Technological
Advancements is a key factor marking the Growth of Achondroplasia Therapeutics
Pipeline
It has been
observed that many pharmaceutical companies are adopting advanced technologies
for the development of achondroplasia therapeutics. These technologies are
helpful in the development of drugs as either combination therapies or
single-agent therapies. For instance, RIBOMIC Inc.’s, RiboART system, is the
essential element in drug discovery technology, that helps in the discovery of
various new drugs for the treatment of the various diseases including
achondroplasia.
Some of the
key players involved in the development of achondroplasia therapeutics include
BioMarin Pharmaceutical Inc., Ascendis Pharma A/S, and Therachon AG.
Achondroplasia
Therapeutics Pipeline Analysis
By Phase
By Molecule
Type
By Route of
Administration
By Company
Tardive Dyskinesia Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations, Collaborations
Pramod Kmr00:28and Other Developments, Clinical Trials & Results, Collaborations, Designations, Patents, Tardive Dyskinesia Therapeutics - Pipeline Analysis
No comments

Tardive
dyskinesia (TD)
is an involuntary neurological movement disorder caused by the use of dopamine
receptor blocking drugs which are prescribed to treat certain psychiatric or
gastrointestinal conditions. Long-term use of these drugs may produce
biochemical abnormalities in the area of the brain known as the striatum.
Access
Detailed Report Summary:
The reasons
that some people who take these drugs may get TD, and some people do not, is
unknown. Tardive dystonia is a more severe form of TD in which slower twisting
movements of the neck and trunk muscles are prominent.
Request
to Get the Sample Pages at:
The typical
symptoms of TD include facial grimacing, sticking out the tongue, and sucking
or fish-like movements of the mouth. INGREZZA (valbenazine), developed by
Neurocrine Biosciences Inc., is one of the key drugs developed for the
treatment of TD.
The drug
candidates in TD pipeline include, but not limited to, SOM3355 and MT-5199.
Some of the companies having drugs in the TD pipeline includes SOM INNOVATION
BIOTECH, SL, and Mitsubishi Tanabe Pharma Corporation.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Major Depressive Disorder Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents
Pramod Kmr00:21and Other Developments, Clinical Trials & Results, Collaborations, Designations, Major Depressive Disorder Therapeutics - Pipeline Analysis, Patents
No comments

Major
depressive disorder, also known as the clinical depression, is characterized by a constant
sense of despair and hopelessness. It usually affects daily activities such as
study, sleep, and eating.
Access
Detailed Report Summary:

Common
symptoms observed in major depressive disorder are fatigue, indecisiveness,
impaired concentration, feelings of worthlessness or guilt, insomnia or
hypersomnia, restlessness, significant weight loss or gain and recurring
thoughts of death or suicide.
Request
to Get the Sample Pages at:
According to
the National Institute of Mental Health, an estimated 16.1 million adults aged
18 or older in the U.S. had at least one major depressive episode in 2014.
VistaGen Therapeutics
Inc. is developing AV-101, a new generation oral antidepressant, for the
treatment of major depressive disorder. NeurOp Inc., and ACADIA Pharmaceuticals
Inc. are some other key players involved in the development of drugs for the
treatment of major depressive disorder.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Lewy Body Dementia Therapeutics - Pipeline Analysis, Clinical Trials & Results, Patents, Designations
Pramod Kmr00:17and Other Developments, Clinical Trials & Results, Collaborations, Designations, Lewy Body Dementia Therapeutics - Pipeline Analysis 2018, Patents
No comments

Lewy
body dementia is
a progressive brain disorder in which Lewy bodies build up in different parts
of the brain that regulate cognition, movement and behavior. Lewy bodies are
abnormal deposition of a protein called alpha-synuclein. The symptoms associated
with the Lewy body dementia includes memory and thinking problems; moving
problems; and sleeping and behavioral changes. It can be treated by
medications, physical therapy, speech therapy, occupational therapy, and
individual and family psychotherapy.
Access
Detailed Report Summary:
Axovant
Sciences Ltd. is in the process of developing a combination of RVT-103 and
RVT-104 for the treatment of Lewy body dementia. Asceneuron SA, and Arena
Pharmaceuticals Inc. are some other key players involved in the development of
drugs for the treatment of Lewy body dementia.
Request
to Get the Sample Pages at:
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Ischemia Reperfusion Injury Therapeutics - Pipeline Analysis, Clinical Trials & Results, Patents, Designations, Collaborations
Pramod Kmr03:24and Other Developments, Clinical Trials & Results, Collaborations, Designations, Ischemia Reperfusion Injury Therapeutics - Pipeline Analysis 2018, Patents
No comments

Ischemia
reperfusion injury is a medical condition which involves tissue injury, resulting in the interruption
of blood supply, and occurs when the blood supply to an area of tissue is cut
off. Ischemia-related tissue injury can occur in various organs including heart
and brain.
Access
Detailed Report Summary:
During
reperfusion injury, inflammatory responses occur including release of free
radicals, vascular leakage, recruitment of inflammatory cells, and proteins
that cause tissue destruction. Currently there are no available drug therapies
but surgeries are available to remove the blood clot responsible for restricted
blood flow.
Request
to Get the Sample Pages at:
Radikal
Therapeutics is in the process of developing R-190 as a new chemical entity for
the treatment of limb ischemia reperfusion injury. Some of the companies having
a pipeline of ischemia reperfusion injury include Catalyst Biosciences Inc. and
NovelMed Therapeutics Inc. among others.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Infertility Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr03:19and Other Developments, Clinical Trials & Results, Collaborations, Designations, Infertility Therapeutics - Pipeline Analysis 2018, Patents
No comments

Infertility is defined as the biological
inability to conceive or contribute to conception, after having regular
unprotected sex. According to the data published by the Department of Health
and Human Services, approximately 10% to 15% of couples in the U.S. are
infertile and have not conceived after at least one year of regular,
unprotected sex.

Access
Detailed Report Summary:
Infertility
may have a single cause in one of the partners, or it could be the result of a
combination of factors. Some of the causes of infertility are ovulation
disorders in women, chemotherapy, radiotherapy, use of illegal drugs, and
testicular infections in men.
Request
to Get the Sample Pages at:
Risk factors
associated with infertility are smoking, alcohol consumption, obesity, eating
disorders, over-exercising, inactivity, sexually transmitted infections (STIs),
mental stress, and exposure to some chemicals. Ferring International Center
S.A. is in the process of developing FE 999310, for the treatment of
infertility. Some of the companies having a pipeline of infertility include
CellOxess LLC, TocopheRx, and others.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hypoxic-Ischemic Encephalopathy Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr03:16and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hypoxic-Ischemic Encephalopathy Therapeutics - Pipeline Analysis, Patents
No comments

Hypoxic-ischemic
encephalopathy
is a serious birth complication that causes obstruction in blood flow in brain
during the prenatal, intrapartum or postnatal period. This leads to death of the
child or mental disabilities in the first two years of age.
Access
Detailed Report Summary:
Although,
the cause of the disease is not yet identified but some conditions like cord
prolapse, abruptio placenta, placenta previa, uterine rupture, breech
presentation, maternal hypotension, or shoulder dystonia are known to be
observed in the patients with this disease. Some of the symptoms observed in
children with this disease are low heart rate, bluish skin colour, poor muscle
tone, and excessive acid in the blood.
Request
to Get the Sample Pages at:
Celgene
Corporation is in the process of developing HPDSC as a cell therapy for the
treatment of hypoxic-ischemic encephalopathy. Assiut University is in the
process of developing erythropoietin for the treatment of hypoxic-ischemic
encephalopathy. Further, GW Pharmaceuticals is also involved in the pipeline
for hypoxic-ischemic encephalopathy.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hypoxia Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents
Pramod Kmr03:12and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hypoxia Therapeutics - Pipeline Analysis 2018, Patents
No comments

Hypoxia is a condition characterized by
inability of the tissues to receive an adequate oxygen supply. This condition
is categorized into four types; hypoxic, stagnant, anaemic, fulminating, and
histotoxic hypoxia. Hypoxic hypoxia is defined as the lack of oxygen in the
arterial blood while in stagnant hypoxia, the rate at which blood circulates in
the body is decreased.
Access
Detailed Report Summary:
Fulminating
hypoxia is an induced type of hypoxia through inhalation of undiluted inert
gases. The lack of the haemoglobin causes less intake of oxygen in the blood,
resulting in the development of anaemic hypoxia. Histotoxic hypoxia occurs due
to cyanide poisoning, in which the tissues are unable to accept oxygen from
capillaries.
Request
to Get the Sample Pages at:
Global Blood
Therapeutics Inc. is in the process of developing GBT440 as an abnormal
haemoglobin modulator for the treatment of hypoxia. NuvOx Pharma LLC is in the
process of developing NVX-108 for the treatment of hypoxia. Akebia Therapeutics
Inc. is also involved in the pipeline for Hypoxia.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hypoxia-Inducible Factor (HIF) Inhibitor Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents,
Pramod Kmr02:20and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hypoxia-Inducible Factor (HIF) Inhibitor Therapeutics - Pipeline Analysis 2018, Patents
No comments

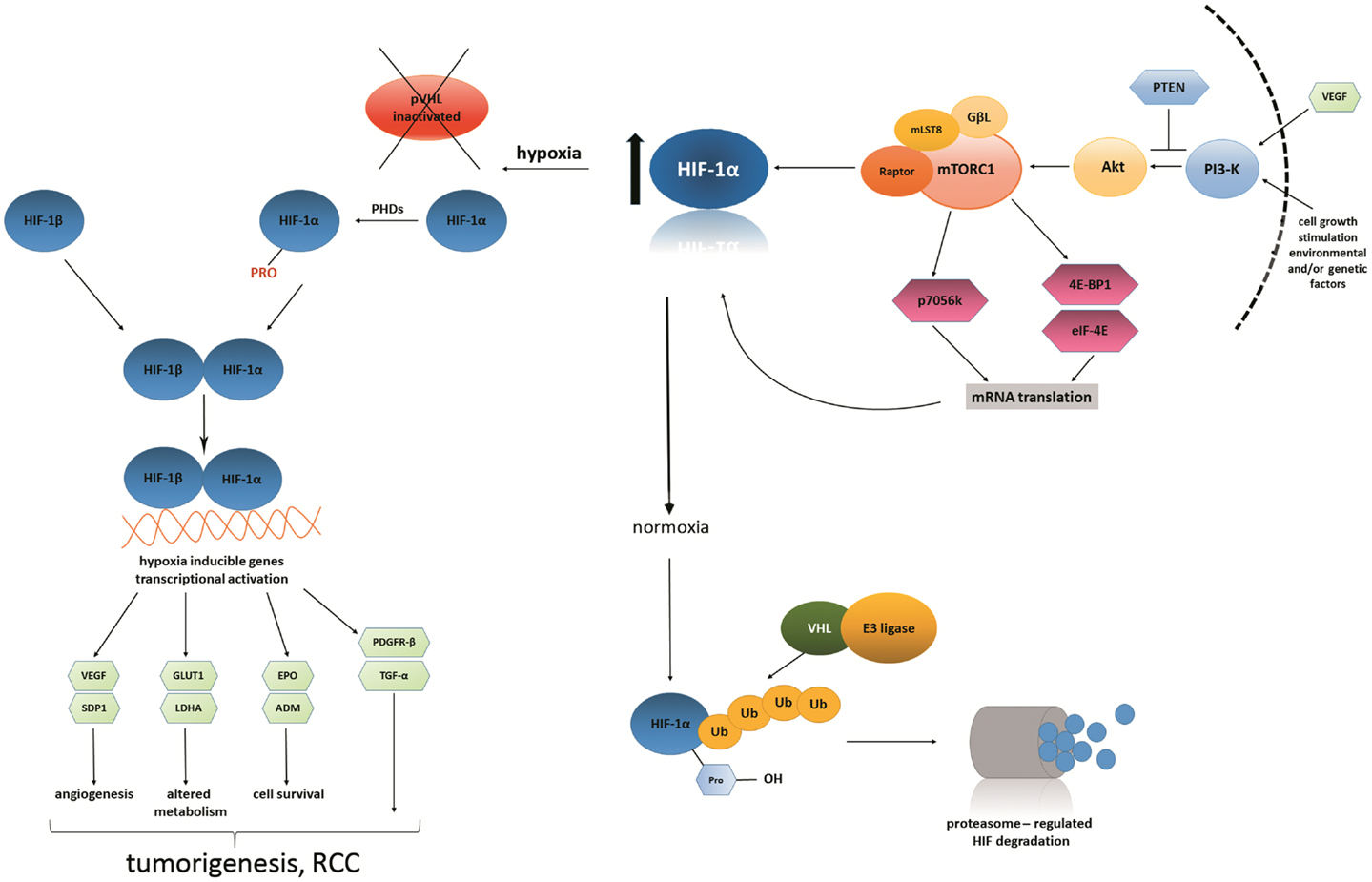
Hypoxia-inducible
factor (HIF) inhibitor inhibits the HIF mediated hypoxic responses. HIF is a transcription
factor that activate when there is a decrease in the availability of oxygen in
the cellular environment. Hypoxia promotes the formation of blood vessels and
this homeostatic system is required in formation of vascular system in the
embryo.
Access
More About This Research at:
In
conditions like wounds, hypoxia mediate the migration of keratinocytes and
restores the epithelium. According to the researchers, the HIF plays a vital role
in the development of human body, as they are required by the body to adapt in
low oxygen concentration and maintain the metabolism. The therapeutic candidate
which acts as an HIF inhibitor has been found to improve the symptoms in
anemia, inflammatory disorders and cancer.
Request
to Get the Sample Pages at:
In addition,
HIF inhibitor have also been found to increase the erythropoietin expression
and enhance hippocampal memory. In addition, HIF is also essential for
immunological responses and is a key physiological regulator of homeostasis,
vascularization, and anaerobic metabolism.
Company like
FibroGen Inc. is developing roxadustat as a HIF prolyl hydroxylase inhibitor
for the treatment of anemia in chronic kidney disease (CKD) and myelodysplastic
syndrome (MDS).
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hyperphosphatemia Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr02:13and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hyperphosphatemia Therapeutics - Pipeline Analysis, Patents
No comments

Hyperphosphatemia is a medical condition which
involves increased level of serum phosphate concentration in the blood.
Phosphate is required for bone and cell formation, genetic coding and energy
metabolism.
Access
More About This Research at:

An
individual is said to have hyperphosphatemia, if the phosphate concentration
increasing beyond > 4.5 mg/dL (> 1.46 mmol/L). Similar to calcium, vitamin D is required to
properly absorb phosphate. The various causes of hyperphosphatemia include
excessive intake of phosphate, inadequate excretion of phosphates,
hypothyroidism, high vitamin D levels, and injuries causing muscle damage.
Request
to Get the Sample Pages at:
Signs and
symptoms associated with the hyperphosphatemia include renal osteodystrophy,
secondary hyperparathyroidism and ectopic calcification. Hyperphosphatemia can
be treated with phosphate binders, vitamin D analogs, and dietary restriction
of phosphate.
Chugai
Pharmaceutical Co. Ltd. is developing EOS789 as an oral formulation for the
treatment of hyperphosphatemia. Some of the other companies developing
hyperphosphatemia therapeutics include Ardelyx Inc., and OPKO Health Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hormone Refractory (Castration Resistant, Androgen-Independent) Prostate Cancer Therapeutics
Pramod Kmr02:09and Other Developments, Androgen-Independent) Prostate Cancer Therapeutics - Pipeline Analysis, Clinical Trials & Results, Collaborations, Designations, Hormone Refractory (Castration Resistant, Patents
No comments

Prostate
cancer occurs
when cells in prostate glands grow uncontrollably. Prostate is an exocrine
gland that makes the fluid part of semen and lie below the bladder in front of
the rectum. As per the National Institute of Health (NIH), 10.7% of all new
cases of cancer in the U.S. are of prostate cancer.
Access
More About This Research at:
https://www.pharmaproff.com/report/hormone-refractory-prostate-cancer-therapeutics-pipeline-analysis
Moreover,
according to the National Cancer Institute, prostate cancer is the second
leading cause of cancer deaths in men. The symptoms of the disease include
frequent urination, difficulty in holding urine, painful or burning sensation
while urinating, blood in the urine, blood in semen, erectile dysfunction, and
pain in the lower back or pelvis or thighs. The treatment options for prostate
cancer include hormone therapy, chemotherapy and vaccine treatment.
Request
to Get the Sample Pages at:
The drug candidates
of hormone refractory (castration resistant, androgen-independent) prostate
cancer pipeline include, but not limited to, AT-001 and Rucaparib. Some of the
companies having drugs in the hormone refractory (castration resistant,
androgen-independent) prostate cancer pipeline includes GTx Inc., Immunomedics
Inc., and Clovis Oncology Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hormonal Replacement Therapeutics - Pipeline Analysis 2018, Clinical Trials & Results, Patents, Designations
Pramod Kmr01:25and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hormonal Replacement Therapeutics - Pipeline Analysis 2018, Patents
No comments

Hormonal
replacement therapy is a type of treatment wherein patients receive a course of hormones in
order to get relieve from the menopause related problems. The therapy involves
administration of synthetic estrogen and progesterone to overcome decreasing
hormone level of women.
Access
More About This Research at:
The most
common signs and symptoms observed in patients with decreased hormone level are
irregular menstrual cycles, and psychological symptoms (mood swings, anxiety,
and urogenital symptoms). Venous thromboembolism, stroke, breast cancer, Alzheimer’s
disease and gall bladder related disorders are some of the major adverse
effects of hormonal replacement therapeutics.
Request
to Get the Sample Pages at:
Evestra Inc.
is developing EC508 hormone replacement therapy for fertility related problems.
Novo Nordisk A/S is another key player involved in development of hormone
replacement therapy.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Heterozygous Familial Hypercholesterolemia (heFH) Therapeutics - Pipeline Analysis , Clinical Trials & Results
Pramod Kmr01:20and Other Developments, Clinical Trials & Results, Collaborations, Designations, Heterozygous Familial Hypercholesterolemia (heFH) Therapeutics - Pipeline Analysis, Patents
No comments

Heterozygous
familial hypercholesterolemia (HeFH) is an inherited genetic disorder that affects the body’s
ability to control cholesterol. It is characterized by very high LDL (low
density lipoprotein) cholesterol (above 190 for adults or above 160 for children)
and family history of high cholesterol, heart disease or stroke.
Access
More About This Research at:
A very high
level of LDL from birth leads to a twenty-fold increase in the risk of
premature cardiovascular diseases. About 1 in 250 people around the world have
HeFH and only about 10% of people with this medical condition have been
diagnosed.
Request
to Get the Sample Pages at:
Diagnosis is
important because there are several early aggressive treatments available which
can lower the risk for heart diseases or stroke.
The drug
candidates of HeFH pipeline include, but not limited to, GEM-201 and
anacetrapib. Some of the other companies having drugs in the HeFH pipeline
includes Gemphire Therapeutics Inc. and Merck & Co. Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Hematopoietic Stem Cell (HSC) Mobilization Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr01:38and Other Developments, Clinical Trials & Results, Collaborations, Designations, Hematopoietic Stem Cell (HSC) Mobilization Therapeutics - Pipeline Analysis, Patents
No comments

Mobilization is the recruitment of hematopoietic
stem cells (HSC) into peripheral blood from the bone marrow following
chemotherapy treatment. There are several advantages for reinfusion of
autologous mobilized peripheral blood stem cells over bone marrow HSC.
Access
More About This Research at:
Some of the
major advantages include enhanced immune reconstitution, shorter duration of
granulocytopenia, shorter hospital stays, reduced morbidity and mortality and
saving of financial resources.

Request
to Get the Sample Pages at:
Several
factors, such as type and dose of cytokines, age, mobilizing chemotherapy
regimen, interval from last chemotherapy cycle and type of previous
chemotherapy cycles or radiation, affects HSC mobilization. BioLineRx Ltd. is
developing BL-8040, a short peptide which acts as a CXCR4 antagonist, for the
treatment of HSC mobilization. Celldex Therapeutics Inc., and Aviara
Pharmaceuticals Inc. are some other key players having pipeline drugs for HSC
mobilization.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Globoid Cell Leukodystrophy (Krabbe Disease) Therapeutics - Pipeline Analysis, Clinical Trials & Results,
Pramod Kmr01:30and Other Developments, Clinical Trials & Results, Collaborations, Designations, Globoid Cell Leukodystrophy (Krabbe Disease) Therapeutics - Pipeline Analysis, Patents
No comments

Globoid
cell leukodystrophy, also known as Krabbe disease, is a genetic disorder which is
characterized by decreased production of galactocerebrosidase. The symptoms
observed during Krabbe disease are fever, vomiting, loss of head control,
irritability and excessive crying, seizures, poor coordination of movement or
stiffness, muscle spasms, changes in muscle tone, deterioration of motor
function, difficulty walking and muscle weakness.

Access
More About This Research at:
There is no
cure for Krabbe disease, however, hematopoietic stem cell transplant (HSCT) has
demonstrated some positive effects on the disease. Moreover, patient’s quality
of life can be improved by anticonvulsant medication to stop seizures, muscle
relaxer drugs, physical therapy to help slow deterioration of muscles and
occupational therapy.
Request
to Get the Sample Pages at:
MediciNova
Inc. is developing MN-166 (ibudilast) as a novel, orally bioavailable small
molecule compound which exerts its effects through several mechanisms to
produce its anti-fibrotic and anti-inflammatory activity for the treatment of
Krabbe disease. BioXcel Corporation, and Zymenex A/S are some other key players
involved in the development of therapeutics for Krabbe disease.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline analysis
of drugs by phases includes product description and development activities
including information about clinical results, designations, collaborations,
licensing, grants, technology, and others.
Female Sexual Dysfunction (FSD) Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr01:18and Other Developments, Clinical Trials & Results, Collaborations, Designations, Female Sexual Dysfunction (FSD) Therapeutics - Pipeline Analysis, Patents
No comments

Female
sexual dysfunction (FSD) prevents the females to experience satisfaction from the
sexual activity. FSD can be developed during any phase of the sexual response
cycle which consists of excitement, plateau, orgasm and resolution phases.
Access
More About This Research at:
Sexual
dysfunction is more common in women than in men. There are several medical
conditions including heart diseases, diabetes, hormonal imbalances,
neurological diseases, kidney diseases, alcohol consumption, depression,
anxiety and drug abuse that leads to FSD.
Request
to Get the Sample Pages at:
There are
several problems associated with FSD such as inhibited sexual desire, inability
to become aroused, lack of orgasm and painful intercourse. S1 Biopharma Inc. is
developing LOREXYS as an oral, non-hormonal, fixed-dose combination of two
antidepressants (bupropion and trazodone) for the treatment of hypoactive
sexual desire disorder (HSDD) in women. Acerus Pharmaceuticals Corporation, and
Palatin Technologies Inc. are some other key players having pipeline products
for the treatment of FSD.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Female Contraception Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr01:15and Other Developments, Clinical Trials & Results, Collaborations, Designations, Female Contraception Therapeutics - Pipeline Analysis, Patents
No comments

Female
contraception
prevents unwanted or unintended pregnancy. When the matured egg cell leave the
ovary and ovulation begins, the chances of becoming pregnant increases. The
main advantage of using contraceptives is reduction in menstrual bleeding and
period pain.
Access
More About This Research at:
Non-hormonal
contraceptives have less side-effects in women as compared to hormonal
contraceptives. Some of the disadvantages observed in women using hormonal
contraceptives are sore breast, nausea, headache, and thrombosis.
Request
to Get the Sample Pages at:
Agile
Therapeutics Inc. is in the process of developing AG200 as an estrogen receptor
agonist for female contraception. Viramal Limited is another key player
developing therapeutics for female contraception related medical conditions.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Epidermolysis Bullosa (EB) Therapeutics - Pipeline Analysis , Clinical Trials & Results, Patents, Designations
Pramod Kmr00:29and Other Developments, Clinical Trials & Results, Collaborations, Designations, Epidermolysis Bullosa (EB) Therapeutics - Pipeline Analysis 2018, Patents
No comments

Epidermolysis
bullosa (EB), is
a rare genetic connective tissue skin disorder that causes blisters and allows
skin to become fragile. Blisters and areas of skin loss (erosions) occur in
response to minor injury or friction, such as rubbing or scratching. The
disease affects 1 out of every 20,000 births in the U.S. The symptoms of
epidermolysis bullosa are redness and heat around an open area of skin;
crusting on the wound surface; pus or yellow fluid discharge; red line or
streak under the skin that spreads away from the blistered area; wound; and
fever or chills.
Request
to Get the Sample Pages at:
Epidermolysis
bullosa can be diagnosed through skin biopsy, prenatal and genetic testing. In
order to treat the disease, surgical treatment or rehabilitation therapy can be
opted. It can also be managed by daily wound care, pain management, and
protective bandaging.
Access
Detailed Report Summary:
The drug
candidates of epidermolysis bullosa pipeline include, but not limited to, EB
101, FCX 007, and Diacerein. Some of the companies having drugs in the
epidermolysis bullosa therapeutics pipeline includes Abeona Therapeutics Inc.,
TWi Pharmaceuticals Inc. and Fibrocell Science Inc.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
Dental Pain Therapeutics - Pipeline Analysis, Clinical Trials & Results
Pramod Kmr23:28and Other Developments, Clinical Trials & Results, Collaborations, Dental Pain Therapeutics - Pipeline Analysis, Designations, Patents
No comments

Dental
pain, also known
as odontogenic pain or tooth pain, arises from the teeth or their supporting
structures which includes mucosa, gingivae, maxilla, mandible, or periodontal
membrane.
Request
to Get the Sample Pages at: https://www.pharmaproff.com/request-sample/1129

The primary
location of the reflected pain (orofacial pain) is the spinal core of
trigeminal system. The trigeminal nerve provides sensory and motor innervation
to the scalp, face, and mouth. Pulpal pain, periodontal pain, gingival pain,
and bone pain are different types of pain originates from the dental area.
Moreover, post-endodontic surgery pain, one of the severe types of pain, occurs
due to apicectomy or root canal therapy.
Access
Detailed Report Summary: https://www.pharmaproff.com/report/dental-pain-therapeutics-pipeline-analysis
Pericoronitis
is an inflammation of soft tissues and is the common cause for removal of
wisdom teeth. Percussion test, probing, pulp sensitivity test, palpation,
mobility test, sinus formation, and radiographic examination are some of the
most common test used to diagnose dental problems.
Stony Brook
University, and Antibe Therapeutics Inc. are some of the key companies and
universities involved in development of dental pain therapeutics. Antibe
Therapeutics Inc. is developing ATB-352 for the treatment of dental pain.
The report
provides a comprehensive understanding of the pipeline activities covering all
drug candidates under various stages of development, with the detailed analysis
of pipeline and clinical trials.
Pipeline
analysis of drugs by phases includes product description and development
activities including information about clinical results, designations,
collaborations, licensing, grants, technology, and others.
















